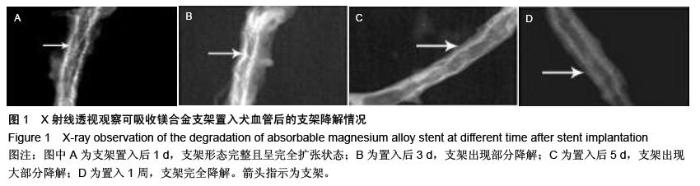
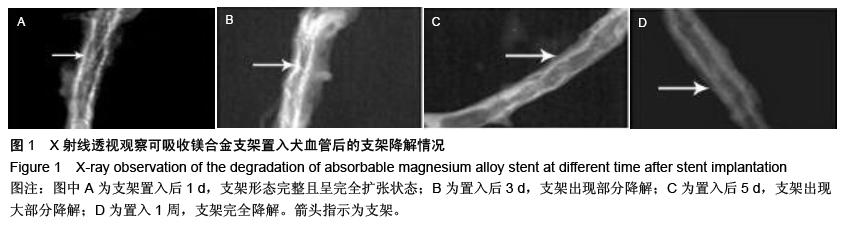
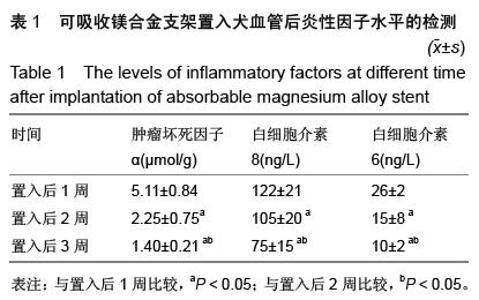
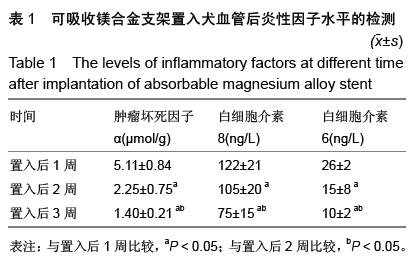
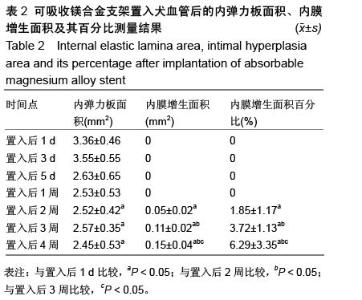
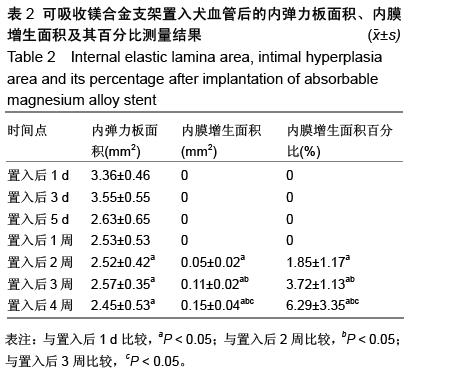
| [1] 陈华,赵仙先.生物可降解镁合金支架研究现状[J].介入放射学杂志,2011,20(1):62-64.
[2] 张朝勇.生物可吸收镁合金药物支架研究进展[J].广西医科大学学报,2012,29(3):483-485.
[3] 易雷,周倩,蒋小莹,等.镁合金可降解血管支架发展历史与研究现状[J].医学与哲学,2015,(6):60-62,91.
[4] 杨水祥,雷力成,王丽丽,等.新型血管内可吸收镁合金支架的实验研究[J].中华老年心脑血管病杂志, 2012,14(7):743-747.
[5] 刘斌,李淑梅,张基昌,等.生物可降解高分子材料载内皮前体细胞CD34抗体涂层支架防治犬冠脉再狭窄的研究[J].中国实验诊断学,2007,11(3):369-371.
[6] 于善坤.医用稀土镁合金支架基体材料制备及力学性能的研究[D].兰州理工大学,2010.
[7] 杨水祥,杨诺,徐桂玉等.新型血管内可吸收镁合金支架的实验研究[C].//第四届国际心血管热点论坛暨心脏交叉学科论坛•北京2012论文集,2012:175-181.
[8] 罗彤.新型可降解聚合物涂层雷帕霉素洗脱支架及镁合金全降解雷帕霉素洗脱支架的可行性研究[D].北京协和医学院,2008.
[9] 高家诚,王强,彭建,等.医用Mg-RE合金血管内支架的研究进展[C].//材料导报,2007:132-135.
[10] 韩欣宇.生物可降解镁合金支架研究现状和进展[J].医学综述, 2015,21(6):981-983.
[11] 胡志勇,王文雯,冯海全,等.镁合金冠脉支架扩张力学性能模拟研究[J].功能材料,2014,45(z1):132-137.
[12] 申祥,倪中华.生物可降解镁合金支架的扩张性能[J].东南大学学报(自然科学版),2008,38(1):49-53.
[13] 谭志刚,周倩,蒋宇钢,等.生物可降解镁合金血管支架:缺点及未来研究趋势[J].中国组织工程研究, 2015,19(8): 1284-1288.
[14] 章晓波,毛琳,袁广银,等.心血管支架用Mg-Nd-Zn-Zr生物可降解镁合金的性能研究[J].稀有金属材料与工程, 2013, 42(6):1300-1305.
[15] 李海伟,徐克,杨柯,等.可降解AZ31镁合金支架在兔腹主动脉的降解性能研究[J].介入放射学杂志, 2010,19(4): 315-317.
[16] Zhang XB,Yuan GY,Fang XX,et al.Effects of solution treatment on yield ratio and biocorrosion behaviour of as-extruded Mg-2.7Nd-0.2Zn-0.4Zr alloy for cardiovascular stent application.Mater Technol. 2013; 28(3):155-158.
[17] Tian P,Liu X.Surface modification of biodegradable magnesium and its alloys for biomedical applications. Regen Biomater.2015;2(2):135-151.
[18] Ning C,Zhou L,Zhu Y,et al.Influence of Surrounding Cations on the Surface Degradation of Magnesium Alloy Implants under a Compressive Pressure. Langmuir. 2015;31(50):13561-13570.
[19] Durisin M,Reifenrath J,Weber CM,et al.Biodegradable nasal stents (MgF<sub>2</sub> -coated Mg-2 wt %Nd alloy)-A long-term in vivo study.J Biomed Mater Res B Appl Biomater. 2015.doi: 10.1002/jbm.b.33559. [Epub ahead of print]
[20] ?apek J,Kubásek J,Vojtěch D,et al.Microstructural, mechanical, corrosion and cytotoxicity characterization of the hot forged FeMn30(wt.%) alloy.Mater Sci Eng C Mater Biol Appl.2016;58:900-908.
[21] Yue Y,Wang L,Yang N,et al.Effectiveness of Biodegradable Magnesium Alloy Stents in Coronary Artery and Femoral Artery.J Interv Cardiol. 2015;28(4): 358-364.
[22] Nguyen TY,Cipriano AF,Guan RG,et al.In vitro interactions of blood, platelet, and fibroblast with biodegradable magnesium-zinc-strontium alloys.J Biomed Mater Res A. 2015;103(9):2974-2986.
[23] Mostaed E,Vedani M,Hashempour M,et al.Influence of ECAP process on mechanical and corrosion properties of pure Mg and ZK60 magnesium alloy for biodegradable stent applications.Biomatter. 2014;4: e28283.
[24] Perkins J,Xu Z,Smith C,et al.Direct writing of polymeric coatings on magnesium alloy for tracheal stent applications.Ann Biomed Eng.2015;43(5):1158-1165.
[25] Zhao N,Zhu D.Endothelial responses of magnesium and other alloying elements in magnesium-based stent materials.Metallomics.2015;7(1):118-128.
[26] Wang J,Giridharan V,Shanov V,et al.Flow-induced corrosion behavior of absorbable magnesium-based stents.Acta Biomater.2014;10(12):5213-5223.
[27] Gökhan Demir A,Previtali B.omparative study of CW, nanosecond- and femtosecond-pulsed laser microcutting of AZ31 magnesium alloy stents. Biointerphases. 2014;9(2):029004.
[28] Drynda A,Hassel T,Hoehn R,et al.Development and biocompatibility of a novel corrodible fluoride-coated magnesium-calcium alloy with improved degradation kinetics and adequate mechanical properties for cardiovascular applications.J Biomed Mater Res A. 2010;93A(2):763-775.
[29] 闫现政,韩东明,杨瑞民,等.可降解镁合金覆膜支架治疗兔颈总动脉侧壁型动脉瘤的可行性[J].中华放射学杂志, 2015,49(2):138-142.
[30] 邓国庆,禹正杨.医用可降解镁合金的研究进展[J].中国生物医学工程学报,2012,31(5):769-774.
[31] 朱悦琦,程英升,李明华,等.生物可降解支架在良性管腔狭窄成形术中应用的研究进展[J].介入放射学杂志, 2008, 17(9):675-680.
[32] 杨凯,朱悦琦,程英升,等.食管良性狭窄药物镁合金可降解支架研究现状及展望[J].介入放射学杂志, 2015,24(5):452-456.
[33] 杨水祥,杨诺,徐桂玉,等.新型血管内可吸收镁合金支架的实验研究[C].//第四届国际心血管热点论坛暨心脏交叉学科论坛•北京2012论文集,2012:175-181.
[34] 王萍,雷力成,王丽丽,等.冠状动脉内可吸收的镁合金支架[J].中国组织工程研究,2012,16(43):8071-8077.
[35] 包汉梅,刘天军,吕丰,等.Mg-PLGA-rhbFGF复合支架在下肢缺血血运重建中的应用研究[J].国际生物医学工程杂志,2012,35(1):33-36.
[36] Luffy SA,Chou DT,Waterman J,et al.Evaluation of magnesium-yttrium alloy as an extraluminal tracheal stent.J Biomed Mater Res A.2014;102A(3):611-620.
[37] 王汝朋,杨水祥.可吸收镁合金支架植入后犬冠状动脉C-反应蛋白、基质金属蛋白酶9和血管性血友病因子的表达[J].中国组织工程研究,2015,19(8):1216-1222.
[38] Wang J,He Y,Maitz MF,et al.A surface-eroding poly(1,3-trimethylene carbonate) coating for fully biodegradable magnesium-based stent applications: Toward better biofunction, biodegradation and biocompatibility.Acta Biomaterialia. 2013;9(10): 8678-8689.
[39] 李禄丰,刘焕云,赵晓辉,等.可降解冠状动脉支架的应用现状[J].中国组织工程研究,2014,18(8):1270-1276.
[40] 冯海全,陈彦龙,何平基,等.镁合金冠脉支架柔顺性能的模拟研究[J].功能材料,2014,45(7):7099-7103.
[41] Hansi C,Arab A,Rzany A,et al.Differences of platelet adhesion and thrombus activation on amorphous silicon carbide, magnesium alloy, stainless steel, and cobalt chromium stent surfaces.Catheter Cardio Inte. 2009;73(4):488-496.
[42] 夏永辉,任玲,徐克,等.镁合金血管支架置入后内膜增生特点[J].介入放射学杂志,2014,23(2):132-135.
[43] 冯高科,任珊,郑晓新,等.生物全降解冠状动脉支架的研究进展[J].医学综述,2012,18(24):4130-4134.
[44] 郭美卿,范海南,杨志强,等.镁合金支架双重可控释放涂层的制备及药物释放性能研究[J].材料导报,2011,25(10): 12-14,31.
[45] 肖健勇,刘寅.镁合金可吸收金属支架的研究进展[J].天津医药,2011,39(3):285-287.
[46] 丁健,刘利珍,王征宇,等.生物可降解镁合金支架的发展[J].医学综述,2015,21(4):646-647,657.
[47] 李树新.生物可降解镁合金支架在冠状动脉血管中应用进展[J].医学综述,2015,21(2):193-195.
[48] 张祎,吕志前,吴信,等.完全生物可降解冠状动脉支架的研究现况与展望[J].生物医学工程与临床,2010,14(2): 171-175.
[49] 黄楠.生物可降解镁及镁合金冠状动脉支架的研究[D].河北工业大学,2009.
[50] 王金瑞,于良,师建华,等.新型可降解镁合金胆道支架的体外降解规律及力学性能[J].中国组织工程研究, 2014, 18 (25): 3980-3986. |